Health
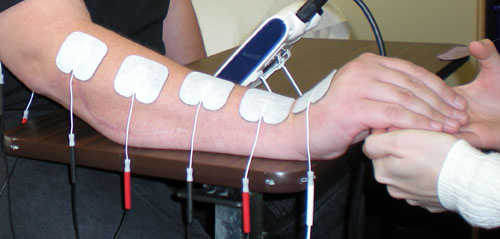
FDA Panel Says No to Shock Therapy Device
[MedPage Today]

GAITHERSBURG, Md. — Members of an FDA advisory panel said Thursday that the agency should ban the use of electrical stimulation devices for aversive conditioning in patients with self-injurious or aggressive behavior.
The majority of the advisers on the agency’s Neurological Devices Panel said the device class poses “an unreasonable and substantial risk of illness or injury” to patients based on all available data and information. Panelists also felt that a clinical study of the device in adults and children would be unethical.
The panel was not asked by the agency to vote on any issue regarding the device, only to discuss a series of questions.
The devices deliver electric shocks and are typically used on severely autistic or developmentally disabled individuals who have repeatedly injured themselves or others through behaviors such as eye gouging, skin pinching, hair pulling, or striking their heads against hard surfaces.